Monitoring in patients with preserved left ventricular function After Diagnosed acute Myocardial Infarction
Terminated study, the number of patients recruited: 4
- Rationale – despite modern optimal therapy, a large proportion of patients remain at high risk for Major Adverse Cardiac Event (MACE) following myocardial infarction
- Implantable cardiac monitors (ICM) have shown up to 80% of patients experience an arrhythmia prior to MACE in MI with reduced EF ≤ 40% – CARISMA trial (n=297, mean 2yr f/u)
- Objective: whether early diagnosis of cardiac arrhythmias, with ICM and remote monitoring, and its consequent treatment, reduce MACE
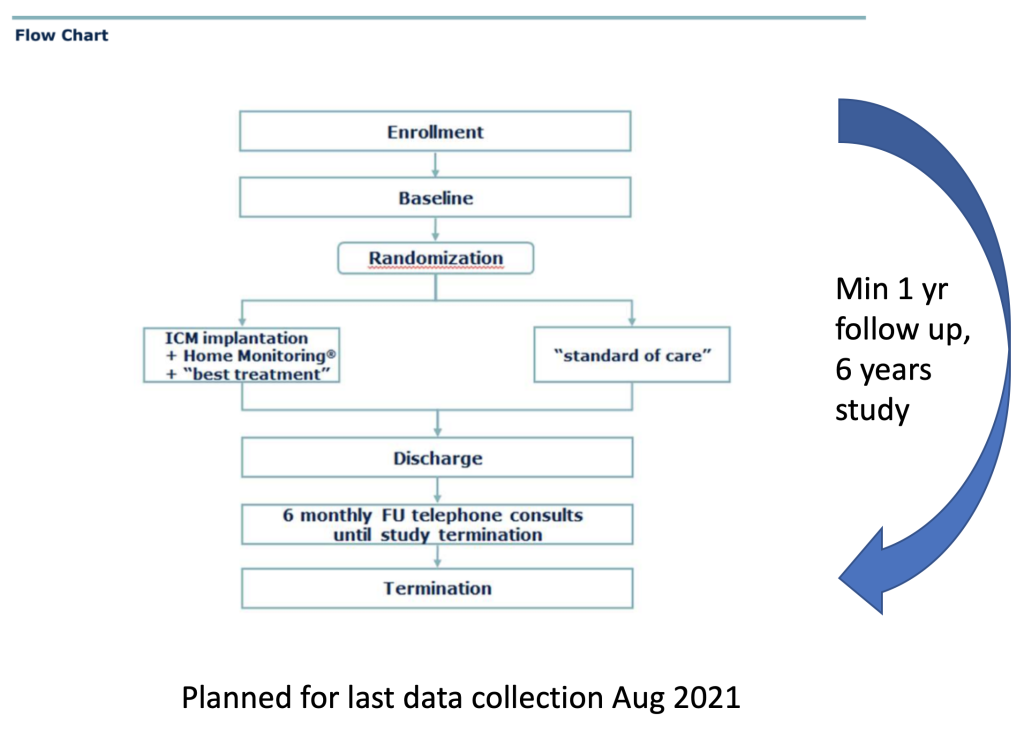
- Prospective, randomised (1:1), parallel-group, open trial;
Primary composite endpoint:
- Time to first MACE – cardiovascular death, first acute unscheduled hospitalisation or urgent visit for worsening of patient status due to heart failure
- First acute unscheduled visit for:
- Arrhythmia
- ACS
- Stroke
- Major bleeding
- Systemic embolism
Secondary endpoints:
- primary composite points separately, WHO-5 well-being index
Inclusion criteria
- History of AMI,
- CHADSVASc ≥ 4 in men, and ≥ 5 in women,
- LVEF > 35% as estimated within 6 months prior to enrolment, but after conclusion of AMI treatment
Exclusion criteria
- Plt < 90,000 per mm 3 or with bleeding diathesis,
- permanent oral anticoagulation for AF,
- indication for renal dialysis,
- indication for PPM,
- Parkinson’s disease,
- life expectancy < 1 yr,
- pregnant or breast feeding
Used systems – BioMonitor or CE-approved successors, Renamic or ICS 3000, home monitoring CardioMessenger II and above with remote assistant