Optimal antiCoagulation for Enhanced risk pAtients Post-catheter ablatioN for atrial fibrillation trial
The number of patients recruited by Canberra Heart Rhythm: 34
Objective: is rivaroxaban superior to aspirin in prevention of stroke for patients post atrial fibrillation catheter ablation.
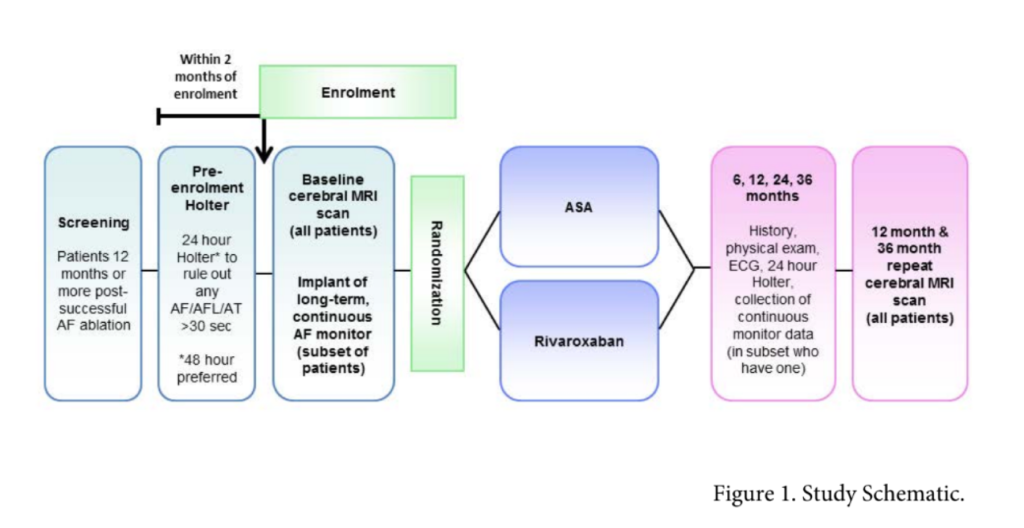
International multi-centre randomised open label study.
Primary endpoint:
Composite of stroke, systemic embolism and covert embolic stroke defined by cMRI
Inclusion Criteria
- Patient must be at least one year post-successful catheter ablation(s) for atrial fibrillation without evidence of any clinically apparent arrhythmia recurrence defined as all of the following:
No AF/AT/AFL on at least 24 hour Holter and an ECG (or equivalent) from 2-6 months after the last ablation, AND no AF/AT/AFL on at least 24 hour Holter and an ECG any time after 6 months after the last ablation AND no AF/AT/AFL on at least 24 hour Holter and ECG 2 months before enrolment in the study. The Holter/ECG within 2 months of enrolment may also serve as the Holter performed 6 months or later after the last ablation - Patient must have a CHA2DS2-VASc risk score of 1 or more. Patients in whom female sex or vascular disease are their sole risk factor may not be enrolled.
- Patient must be >18 years of age.
- Patient must have non-valvular AF.
Exclusion Criteria
- Bleeding contra-indication to oral anticoagulation, or medications with strong interactions with oral anticoagulation
- Non-arrhythmic condition necessitating long-term oral anticoagulation.
- Contraindication to magnetic resonance imaging (MRI) or is unlikely to tolerate due to severe claustrophobia.
- Contraindication to implantation of an implantable loop recorder (such as limited immunocompetence or a wound healing disorder).
- Valvular atrial fibrillation
- Severe, disabling stroke within one year prior to enrollment or any stroke within 14 days of enrollment.
- Special risk factors for stroke unrelated to AF, specifically known thrombophilia/hypercoagulability, uncontrolled hypertension (systolic blood pressure >180 mmHg and/or diastolic blood pressure >100 mmHg within 4 days of enrollment), untreated familial hyperlipidemia, known vascular anomaly (intracranial aneurysm/ arteriovenous malformation or chronic vascular dissection), or known severe carotid disease.
- > 85 years of age.
- Critically ill or who have a life expectancy <3 years.
- Once enrolled, randomisation into either:
- Rivaroxaban 15mg daily
- Aspirin 75-160mg daily
- MRI at baseline and 3 year follow up
- Subset of patients (n=500) will have implantable loop recorder inserted