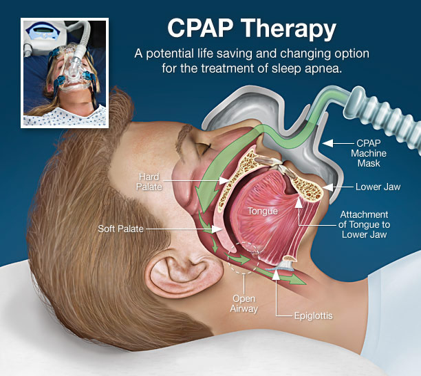
Sleep Apnoea and Atrial Fibrillation: treatment of sleep apnoea, effects on ablation outcomes (SNORE-AF)
The number of patients recruited by Canberra Heart Rhythm: 36 and still enrolling
SYNOPSIS OF THE STUDY:
- The study aims to investigate the impact of Obstructive Sleep Apnoea treatment on Atrial Fibrillation utilising a randomised controlled trial design.
- Hypothesis: treatment of sleep apnoea will reduce the burden/recurrence of atrial fibrillation.
- Primary endpoint: time to AF recurrence following AF ablation at 12 month
-
Secondary endpoint: single procedure freedom from AF recurrence at 24 months, multi-procedure AF recurrence at 12 and 24 months and reduction of AF burden
INCLUSION CRITERIA:
- Has atrial fibrillation
- Moderate to servere obstructive sleep apnoea as AHI > or = 15
- Over 18yrs olf
- Able and willing to provide informed consent and comply with all the testing and requirements
EXCLUSION CRITERIA:
- Has valvular heart disease
- Has AF due to trasient or reversible cause ie. post operative cardiac or non-cardiac surgery lung disease, hyperthyroidism